Turning the Tide on Cancer
Cardiff Oncology is a clinical stage biotechnology company leveraging PLK1 inhibition to develop novel therapies across a range of cancers.
Cardiff Oncology First Quarter 2024 Financial Results and Corporate Updates on May 2, 2024 at 4:30 p.m. ET/1:30 p.m. PT
Our lead asset is onvansertib, a PLK1 inhibitor that we are evaluating in combination with standard of care (SoC) therapeutics in clinical programs targeting indications with the greatest need for new treatment options.
These programs and our broader development strategy are designed to target tumor vulnerabilities to overcome treatment resistance and deliver superior clinical benefit to patients compared to the SoC.
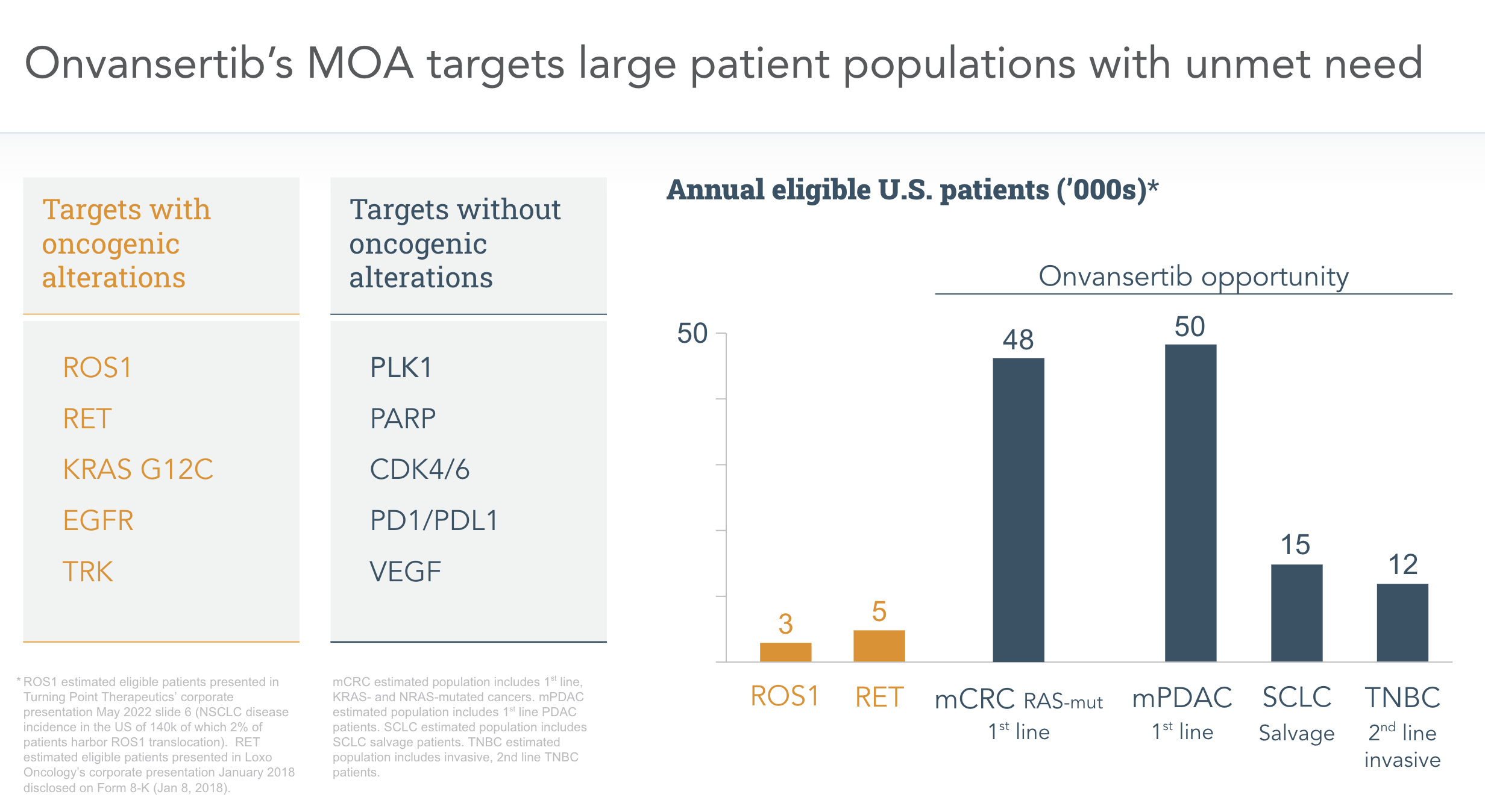
Current
Clinical
Programs
1st Line RAS-Mutated Metastatic Colorectal Cancer (mCRC)
Combining 20mg or 30mg of onvansertib with SoC vs SoC alone (SoC is either FOLFIRI/bevacizumab or FOLFOX/bevacizumab) to demonstrate preliminary safety and efficacy data and confirm an optimal dose.
2nd line KRAS-Mutated Metastatic Colorectal Cancer (mCRC)
Combining onvansertib with FOLFIRI/bevacizumab to improve objective response rate (ORR) and progression-free survival (PFS)
Metastatic Pancreatic Ductal Adenocarcinoma (mPDAC)
Combining onvansertib with nanoliposomal irinotecan and 5-FU to improve objective response rate (ORR) and progression-free survival (PFS)
